2021/11> New EPO guidelines for antibodies
Guidelines for Examination in the European Patent Office (EPO) have been updated in 2021 with a new section dedicated to antibodies (Guidelines G-II, 5.6). This new section explains the EPO requirements to define antibodies in claims (Guidelines G-II, 5.6.1) and addresses the EPO requirements with respect to the inventive step of antibodies (Guidelines G-II, 5.6.2).
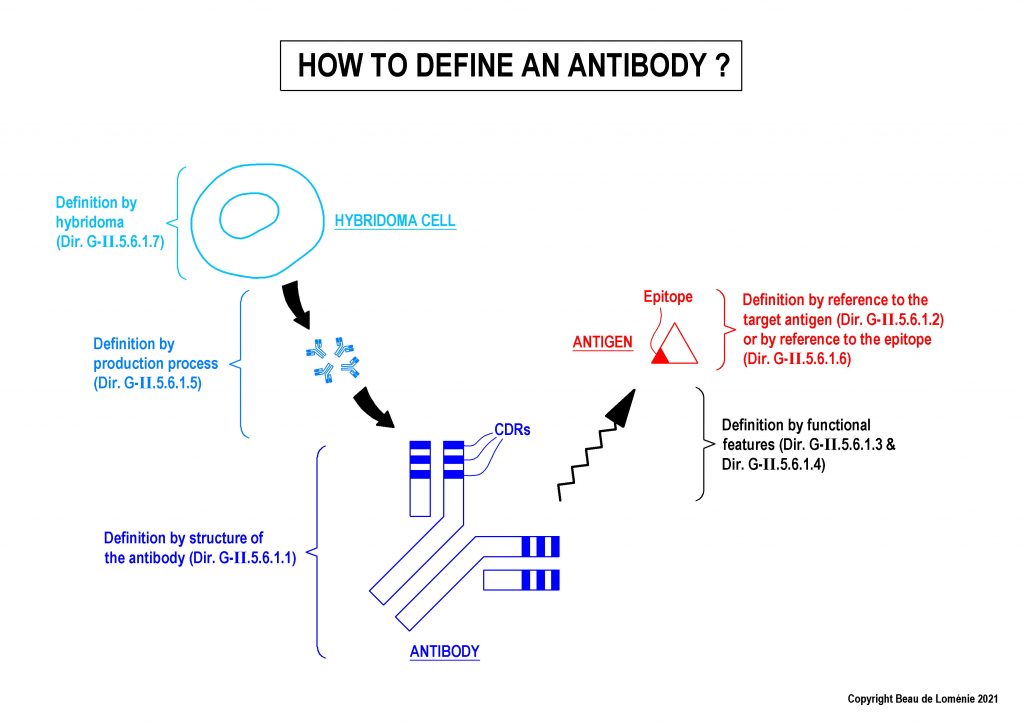
1 – How to define antibodies in claims? (Guidelines G-II, 5.6.1)
The Guidelines indicate that conventional antibodies can be defined by different ways, including (but not limited to) structural features and functional features.
Definition by structure of the antibody
The Guidelines indicate that it is necessary to define a conventional antibody by at least its six CDRs to fulfil the requirements of clarity (Art. 84 EPC).
A claim to an antibody defined by its structure by fewer than six CDRs will be considered to fulfil the requirements of Art. 84 EPC only if it is experimentally shown that one or more of the six CDRs do not interact with the target epitope or if it concerns a specific antibody format allowing for epitope recognition by fewer CDRs.
Definition by functional features (target antigen, epitope, binding features)
It is possible to define the antibody by reference to the target antigen, as long as the antigen is clearly defined in the claims.
The EPO Guidelines do not indicate what would be considered as “clearly” defined. One clue would be to specify the sequence(s) of the antigen in the claims (and in the description) or to ensure that the meaning of the term is clear for the person skilled in the art from the wording of the claim alone. The Guidelines specify that when a protein sequence is introduced, an open language (such as “comprising”) or sequence variability would not be allowed, otherwise the claim will be considered to lack novelty over prior art.
- An antibody may be defined also by its epitope (linear epitope or discontinuous epitope). For linear epitope, it is recommended to define the epitope as a limited fragment using closed wording (e.g. epitope consisting of). If the epitope is “discontinuous”, it is needed to clearly identify the specific amino acid residues of the epitope and the method for determining the discontinuous epitope must also be indicated in the claim.
- Functional features of the antibody can be further introduced in the claims defining the antigen or the epitope by functional properties, such as the binding affinity, neutralising properties, induction of apoptosis, internalisation of receptors, inhibition or activation of receptors.
However, since an antibody defined by functional features and/or by its epitope cannot be easily compared with known antibodies, there is also a risk of implicit disclosure, unless the Applicant demonstrate that antibodies from the prior art do not display the same functional features and/or target another epitope than the one claimed. The burden of proof of novelty to distinguish the claimed antibody from the prior art resides with the applicant.
Definition by hybridoma or by production process
Antibodies can also be defined by the process of their production, i.e. either by the immunisation protocol of a non-human animal with a well-characterised antigen or by the specific cell line used to produce them. However, this definition should be based on the immunisation by an antigen comprising a sequence 100% identical to a defined sequence (and not less than 100%) because the use of variants is considered to render the scope of the antibodies obtained by the immunisation process unclear.
Antibodies may also be defined through a deposited hybridoma cell producing the antibodies.
2 – Sequence identity
The EPO Guidelines confirm that it is possible to claim an antibody characterised by the sequences of both variable domains or CDRs with less than 100% sequence identity when combined with a clear functional feature.
However, the percentage of sequence identity that could be allowed is not specified in the EPO Guidelines. According to our experience, an antibody characterised by the sequences of both variable domains or CDRs with less than 80 % sequence identity generally leads to an objection by the EPO.
3 – Inventive step of antibodies
Under European practice, inventive step can be recognized for a novel, further antibody binding to a known antigen only if a surprising technical effect is shown by the application. Said surprising technical effect should be demonstrated by a comparison with the antibodies known from the prior art and is for example an improved affinity, an improved therapeutic activity, a reduced toxicity or immunogenicity, an unexpected species cross-reactivity or a new type of antibody format with proven binding activity.
The EPO Guidelines indicate (or confirm) important criteria for the assessment of inventive step of antibodies:
- If the surprising effect involves the binding affinity, the structural requirements for conventional antibodies inherently reflecting this affinity must comprise the six CDRs and the framework regions because these regions can influence the affinity.
- If inventive step relies on an improved property versus the enabled antibodies of the prior art, the main characteristics of the method for determining the property must also be indicated in the claim or indicated by reference to the description.
- If a novel antibody binds to the same antigen as known antibodies, inventive step is not acknowledged solely on the basis that the novel antibody is structurally different (e.g. different sequence) from the known antibodies. The EPO Examiner will consider these antibodies as obvious alternative antibodies.
- Antibodies can be inventive if the application overcomes technical difficulties in producing or manufacturing the claimed antibodies.
Our experts in biotech remain at your disposal to answer your questions.